Cookies!
Cookies!

-
Categories
- ATI
- NR
- OCR GCSE Papers & ma...
- AQA papers mark sche...
- Relias Dysrhythmia
- OCR GCE A & AS LEVE...
- OCR GCSE Question Pa...
- Pearson Edexcel A an...
- EXAM
- Summary
- Study Notes
- QUESTION PAPER (QP)
- QUESTIONS & ANSWERS
- CASE STUDY
- Class Notes
- ESSAY
- Presentation
- Report
- Judgements
- Manual
- Summary
- STUDY GUIDE
- Thesis
- Visual
- Text Book Notes
- BUSINESS PLAN
- Syllabus
- LECTURE NOTES
- E-Book
- EXAM PROCTORED
- NCLEX-PN
- NCLEX-RN
- ORDER CUSTOM PAPER H...
- Dissertation
- Research Paper
- DISCUSSION POST
- Final Exam Review
- EXAM REVIEW
- SOAP NOTE
- iHuman
- SHADOW HEALTH
- TEST BANK
- MILESTONE
- HESI
- ATI MEDICAL SURGICAL
- QUESTIONS and ANSWER...
- SOPHIA PATHWAY
- MED-SURG EXAM
- HESI MED SURG
- UWorld
- Lab Experiment
- Lab Report
- Experiment
- NCLEX
- PATIENT ASSESSMENTS
- JOURNAL
- SOPHIA Milestone
- VSIM for NURSING FUN...
- PROJECT FINAL
- CAPSTONE SIMULATION
- VATI RN
- VATI PN
- Portfolio
- GIZMOS
- Solutions Guide
- SOLUTIONS MANUAL
- vSim For Nursing
- SWIFT RIVER
- MARK SCHEME
- Virtual Clinical Exp...
- AQA
- AQA Questions and Ma...
- Higher Education
- Edexcel
- INSTRUCTOR MANUALS
- ATI
- Advanced Trauma Life...
- GUIDELINES
- INTERVIEW
- Object-Oriented Prog...
- AS Mark Scheme
- A-Level Mark Scheme
- ANSWERS AND COMMENTA...
- GCSE MARK SCHEME
- GCSE QUESTION PAPER
- AQA Question Papers
- A/As Level Mark Sche...
- AS Level Mark Scheme
- RESOURCE BOOKLET
- Edexcel Question Pap...
- QUESTION PAPER & MAR...
- Test Prep
- LAB QUIZ
- Quiz
- PREDICTED PAPER
- IGCSE
- Examiners’ Report
- SPECIMEN INSERT
- INSERT CONTENT PAPER
- AQA A/As Level Quest...
- As Level Question Pa...
- Cambridge Internatio...
- Cambridge IGCSE QP
- Cambridge IGCSE MS
- BTEC Nationals
- Edexcel Mark Scheme
- A Level Question Pap...
- AS Level Question Pa...
- CHEAT SHEET
- Capism
- FISDAP
- AHIP
- Feedback Log
- Book Review
- FILM REVIEW
- POEM ANALYSIS
- SUMMARY
- PLAY ANALYSIS
- MOVIE ANALYSIS/REVIE...
- SAT
- LSAT
- MCAT
- TOEFL
- IELTS
- Textual Analysis
- Annotated Bibliograp...
- CODING SOLUTION
- Literature
- COURSE NOTES
- ASSIGNMENT
- PROJECT REPORT
- SOLUTIONS
- EXAM/TEST TEMPLATE
- TEMPLATE
- HOMEWORK
- WORKSHEET
- TEST PREP
- English Literature
- FINAL EXAM
- HESI A2
- APEA
- CAPSTONE
- SIMULATION
- PROGRAMMING
- HTML
- USMLE
- HARVARD CASE SOLUTIO...
- CASE SOLUTIONS
- Exam (elaborations)
- Answers
- Other
- Textbook notes
- Case
- TEST BANKS
- AMLS
- A Level & AS Level N...
- Exam Elaborations
- NRNP
- WGU C214
- Straighterline
- NBME
- NSG
- AQA 2023
- AQA GCSE QUESTION PA...
- Prophecy Pacu
- Prophecy Medical Sur...
- Prophecy RN
- TNCC
- WGU C215
- Texas All Line
- Rasmussen Pharmacolo...
- AQA Papers & Mark Sc...
- EMT BLOCK
- PAX
- EXCEL CRASH COURSE
- EMT FISDAP
- ATI Dosage Calculati...
- APEX
- TMC
- OCR GCSE
- Wonderlic
- VATI
- ANCC
- Smart Serve
- WGU C428
- AQA GCSE COMBINED SC...
- OCR PAPERS & MARK SC...
- NAPRx
- NUTRITION 101
- WGU C207
- USPS
- Support
- Cart {{ cart.length }}
- Account
 View example
View example
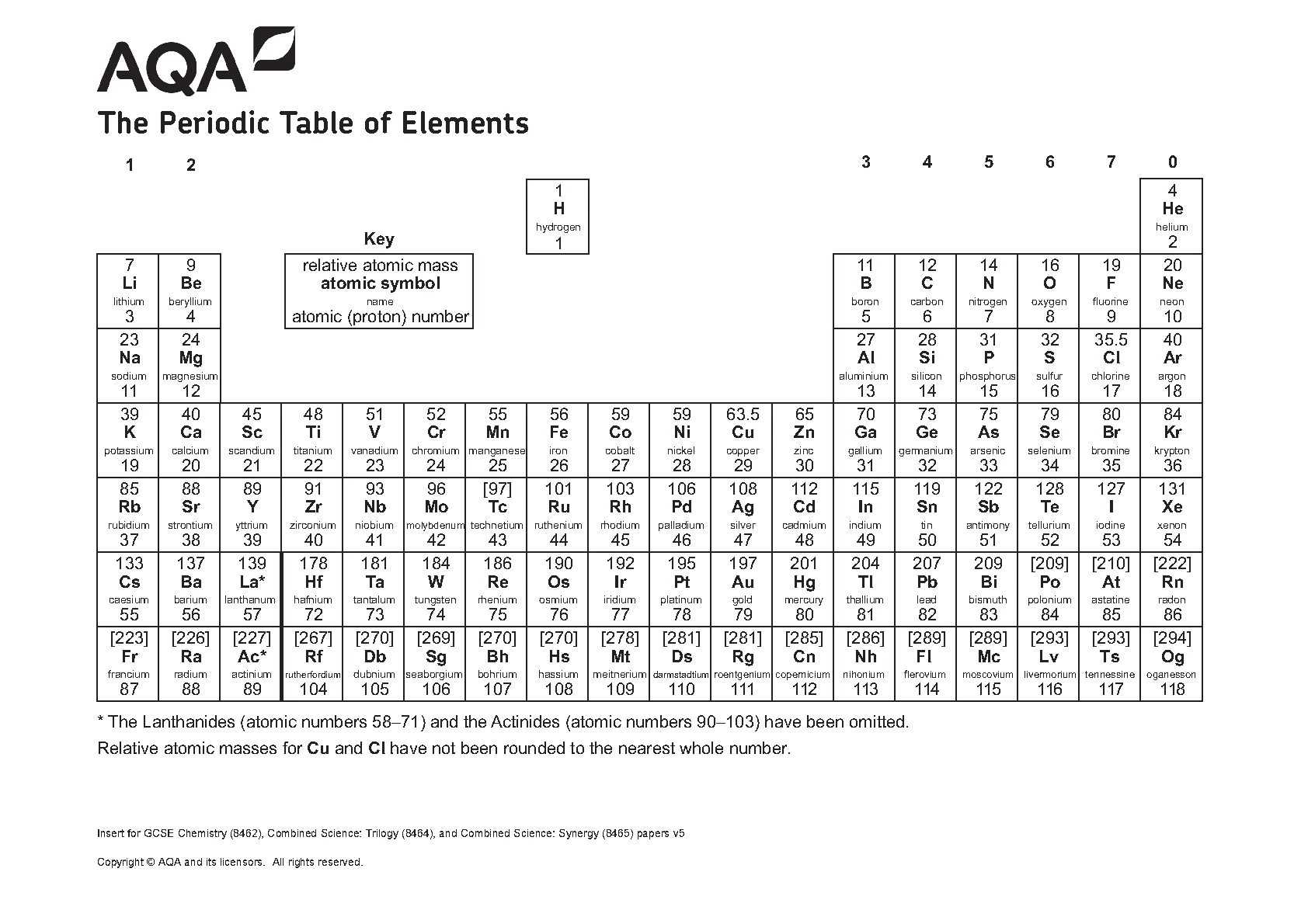
AQA GCSE CHEMISTRY PAPER 2 QUESTION PAPER 2023 (8462/2H: Higher Tier). DOWNLOAD OFFICIAL AND VERIFIED AQA GCSE CHEMISTRY PAPER 2 QUESTION PAPER 2023 ON www.leakedexams.com.
0 1 box A student investigated the rate of the reaction between zinc and sulfuric acid.
Hydrogen gas is produced during this reaction.
Figure 1 shows the apparatus.
Figure 1
This is the method used.
1. Add 50 cm3 of sulfuric acid to a conical flask.
2. Add 2.0 g of zinc to the conical flask.
3. Quickly put a stopper in the conical flask and start a timer.
4. Measure the time taken to collect 20 cm3 of gas.
5. Repeat steps 1 to 4 three more times.
0 1 . 1 Suggest why the stopper must be put in the conical flask as quickly as possible in
step 3.
[1 mark]
| Author | OCR KING |
| Published | 15 Dec 2025 |
| Included files | |
OCR GCE A LEVEL PHYSICS B PAPER 2 MARK SCHEME 2023...
AQA GCSE SOCIOLOGY PAPER 1 QUESTION PAPER 2023 (81...
AQA GCSE COMBINED SCIENCE: TRILOGY INSERT 2023 (84...
2023 AQA GCSE GEOGRAPHY INSERT PAPER 1 (8035/1: Li...
2023 AQA GCSE GEOGRAPHY INSERT PAPER 1 (8035/1: Li...
2024 OCR GCSE BIOLOGY A PAPER 1 MARK SCHEME (J247/...
2024 OCR GCSE Media Studies Insert Paper 1( J200/0...
2024 OCR GCSE Media Studies Paper 1 (J200/01 Telev...
2024 OCR GCSE Media Studies Paper 2 (J200/02 Music...
2024 OCR GCSE Psychology Paper 2 (J203/02 Studies...

 Cookies!
Cookies!